
Organic Chemistry Laboratory I
Acid Catalyzed Isomerization of Carvone to Carvacrol
Experimental Procedure

Organic Chemistry Laboratory I
Acid Catalyzed Isomerization of Carvone to Carvacrol
Experimental Procedure
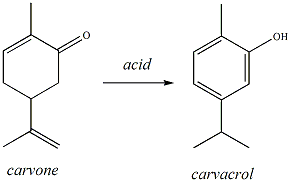
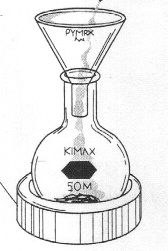
| Synthesis of Carvacrol Clamp a 50ml round-bottomed flask to a ring stand and place the reaction flask into a snug-fitting, 50ml heating mantle. Position the reaction set-up on the side of the hood with the faucet labeled "distillation set-up". Set the heating mantle on a stirrer/hotplate. Using a funnel, dispense 2 ml of carvone and 20ml of 6M H2SO4 into a the flask using a funnel (Figure 2). CAUTION!! 6M H2SO4 is very strong acid. Wear gloves and rinse them under water after handling acid. Add a magnetic stir bar and gently begin to stir the reaction mixture by turning on the magnetic stirrer. Connect the heating mantle to a Variac. Be sure to twist the plug tightly to ensure appropriate connectivity. Insert a condenser tube into the reaction flask and attach the water hoses (in at the bottom, out at the top) (Figure 3). Insert a drying tube into the top of the reflux apparatus. Turn on the water. Plug the Variac into the wall outlet and set the dial to between 50 and 60V. Turn on the Variac and heat the reaction mixture to reflux (i.e, until the contents is boiling and the vapors condense and drip back into the flask) for 35 minutes. |
 Figure
2: Dispensing reagents into rb flask
|
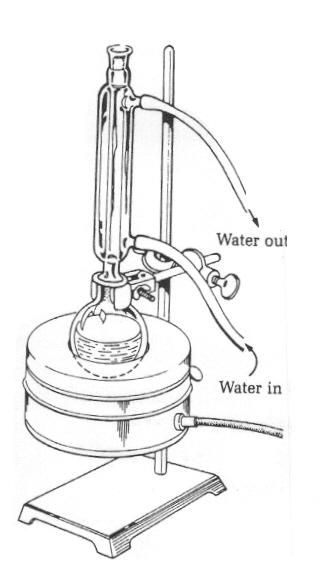
Figure 3: Reflux apparatus
|
|
While
the reaction mixture is refluxing, obtain two, clean and dry 50ml
Erlenmeyer flasks or beakers. Label one as "organic layer" and
the other as "aqueous layer". Set the flasks aside. Reaction Work Up After 35 minutes, turn off the Variac and allow the reaction mixture to cool completely to room temperature. Remove the condenser tube and insert a glass funnel into the neck of the flask. Add 10ml of petroleum ether to the reaction flask and swirl the contents. Transfer the mixture to a 100ml Erlenmeyer flask and label the flask as "crude reaction mixture". Set up a 125ml separatory funnel on a ring stand as shown in Figure 4. Close the stopcock on the separatory funnel and dispense the crude reaction mixture into the separatory funnel using a glass funnel. Allow the mixture to settle. Two layers should be observed. Remove the glass funnel and place the stopper in the top of the separatory funnel. Shake the funnel as illustrated in Figure 5. Return the separatory funnel to the ring stand an allow the layers to separate again. Separate the two layers and dispense each layer into the two clean 50ml beakers or Erlenmeyer flasks prepared earlier, placing the organic layer into the flask labeled "organic layer" and the aqueous layer into the flask labeled "aqueous layer". (To determine which is the organic layer add a few milliliters of petroleum ether to each layer. If the few milliliters is miscible, that's the organic layer; if it separates out, its the water layer.) Return the aqueous layer to the separatory funnel. |
|
 Figure
4: Separatory funnel
|
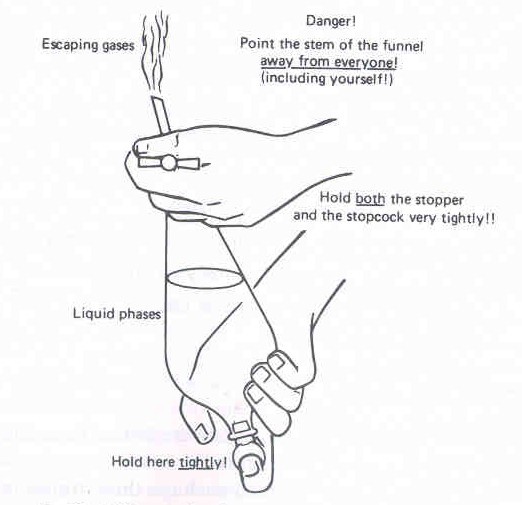 Figure 5: Shaking the separatory funnel |
|
Add an
additional 10ml of
petroleum ether to the separatory funnel, shake again and allow the
layers to settle for a second time. Dispense the organic layer
into the beaker containing the first organic layer. Save the
water layer and set it aside.
Return the combined organic layers to the separatory funnel and add
20ml of 5% NaHCO3.
Shake the separatory funnel as in
the previous steps and allow the layers to separate. Dipsense
each layer into labeled, clean, dry 50ml beakers or Erlenmeyer
flasks.
Label one as "organic layer 2" and the other as "water layer 2".
Add
20-50mg of MgSO4 or Na2SO4 drying
agent to the organic layer and swirl until the drying agent moves
freely in the flask. Filter the mixture to remove the drying
agent and collect the filtrate into a
clean, dry 50ml Erlenmeyer
flask. The filtrate will contain the synthesized product. TLC Analysis of the Reaction Run a TLC of the product in the petroleum ether solution against the starting material. Use silica gel plates as stationary phase and determine an appropriate solvent system for developing the TLC plate, starting with 50:50 hexane: ethyl acetate. Adjust the polarity of the developing solvent to acheive Rf values in the range of 0.2-0.8. Report the specific TLC conditions used to achieve these Rf ranges. |