
ACP Home | Organic Chemistry I | Organic Chemistry II | Lecture | Laboratory | General Chemistry | Laboratory Syllabus
Separation & Purification of
Components of an Analgesic Tablet
Experimental Procedure

ACP Home | Organic Chemistry I | Organic Chemistry II | Lecture | Laboratory | General Chemistry | Laboratory Syllabus
Separation & Purification of
Components of an Analgesic Tablet
Experimental Procedure
Label everything
Do not throw anything away until you are 100%
sure
you have the desired products in your hands
The procedure is provided in a text format. A flowchart format is also available which is a summary of the procedure. Use both of these formats for the most thorough coverage of the experimental procedure. See "Creating and Using Flowcharts for Experimental Procedures" for more details.
Procedure
Grind four Excedrin tablets to a fine powder using a mortar and pestle
(Figure 10). Weigh the solid and record the weight in your
notebook.
Transfer the solid to a 50ml Erlenmeyer flask. Add 15ml of
dichloromethane
(CH2Cl2, also called methylene chloride) and a
stir
bar to the flask. Clamp the flask to a ring stand and set it on a
hot plate/stirrer in the hood. Begin stirring the mixture.
Gently warm the mixture to just below boiling (CH2Cl2
has a very low boiling point, 40°C) for 5-10 minutes. All of
the solid will not dissolve.
 Figure 10: Mortar & Pestle used for Grinding Tablets |
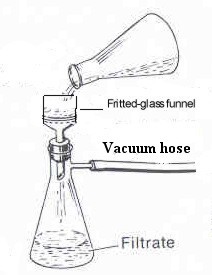
Figure 11: Vacuum Filtration Apparatus |
Filter the solid, while still warm, by vacuum filtration. Set
up a vacuum filtration apparatus as depicted in Figure 11. Clamp
a 125 ml vacuum flask to a ring stand. Insert a neoprene adapter
into the neck of the flask and fit the flask with a Buchner
funnel.
Insert a piece of filter paper into the funnel. wet the filter paper
with
CH2Cl2. Attach a vacuum hose (thick-walled;
Do not use a thin-walled hose. It will collapse under vacuum) to
the flask and the sink aspirator. Pour the warmed mixture
of Excedrin tablets and CH2Cl2 through the
filtration
apparatus and allow the solid to sit in the filter to dry for ~
5minutes
with the vacuum turned on. The solid in the filter contains
tablet
binder (non-active ingredient) and acetaminophen (ACE). Transfer
the solid to a 50ml beaker using a spatula. Label the
beaker
“Binder +ACE” with a permanent marker. Set the beaker aside and
allow
it to dry for the remainder of the lab period.
|
|
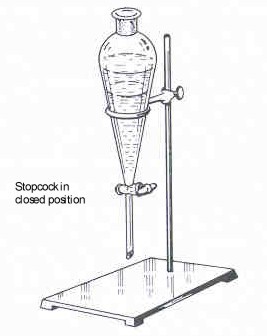 Figure 12: Separatory Funnel Positioned on the Ring Stand (adapted from Landgrebe, p. 124) |
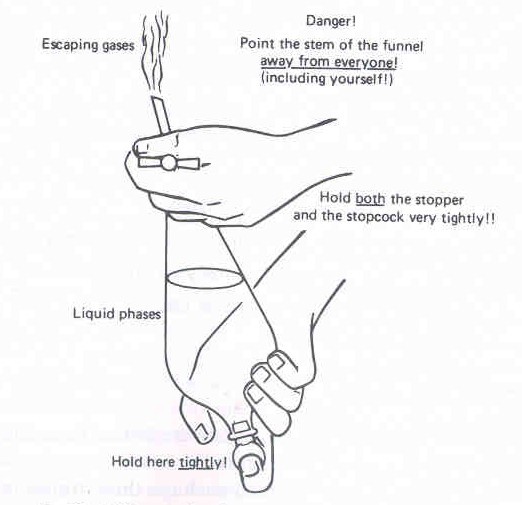
Figure 13: Shaking a Separatory Funnel |
TLC Analysis of ASA and CAF
Add 10 ml (or less) of CH2Cl2 to the ASA and
CAF flasks to completely dissolve any solids. Prepare a silica
gel
TLC plate with five tick marks as described in TLC
Analysis of Analgesics. Label the tick marks 1-5 and
construct
a table in your notebook as shown in Table 3.2, listing what each tick
mark represents. Spot
the plates with each of the two experimental solutions in the
flasks
labeled ASA and CAF and with known solutions of ASA, CAF and ACE. Develop
the plates using the developing solvent that worked best in the TLC
Analysis of Analgesics experiment. When the plate is developed,
remove
it from the developing chamber and immediately mark the solvent front
and
label the plate “ASA & CAF”. View the plate under UV light
and
circle any visible spots. Place the TLC plate in an I2
chamber and observe the appearance of spots. Calculate
Rf values for all spots observed. Tape the plate into the
results
section of your notebook.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 2: TLC Analysis of ASA and CAF
At this point the three active constituents of Excedrin are
separated.
Collect the two flasks, labeled “CAF” and “ASA” and the watch glass
labeled
“Binder + ACE”. Cover the flasks with paraffin and set them in
your
lab drawer until next week. Transfer the solid on the watch glass
to a vial using a spatula. Label the vial “Binder + ACE” and
place
the vial in your lab drawer until the second week of the experiment.
|
|
TLC Analysis and Isolation of ACE
Transfer the solid “Binder + ACE” in the vial to a 50 ml Erlenmeyer
flask, using a spatula. Add a magnetic stir bar and 15 ml of
ethanol
to the flask. Place the flask on a hot plate/stirrer and begin
stirring.
Gently warm the mixture just below boiling for ~10 minutes. (All
of the solid will not dissolve.) Set up a vacuum filtration
apparatus
as shown in Figure
11 and filter the solid (binder) from the filtrate (solution of ACE
in ethanol). Label the vacuum flask containing the filtrate
“ACE”.
Prepare
a TLC plate with 4 tick marks. Label the tick marks 1-4 and
construct
a table in your notebook as shown in Table 3.3, listing what each tick
mark represents. Spot
the plates with the experimental solution of ACE and with known
solutions
of ACE, ASA and CAF. Develop
the plates using the developing solvent that worked best in the TLC
Analysis of Analgesics experiment. When the plate is
developed, remove it from the developing chamber and immediately label
the plate “ACE” and mark the solvent front. View the plate under
UV light and circle any visible spots. Place the TLC plate in an
I2 chamber and observe the appearance of spots. Calculate
Rf values for all spots observed. Tape the plate into the
results
section of your notebook.
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
Table 3: TLC Analysis of ACE
Place the vacuum flask containing ethanol and ACE on a hot
plate/stirrer.
Add a magnetic stir bar, begin stirring and gently heat the
solution
to evaporate ~2/3 of the ethanol. Remove the flask from the heat
and allow it to cool to room temperature. Clamp the flask to a
ring
stand and set the flask in an ice bath. Proceed with the
remainder
of the experiment. Check the mixture after ~30 minutes. A
solid
(ACE) should precipitate from the solution. Carefully decant off
any remaining ethanol. Weigh a watchglass and record the weight
in
your notebook. Transfer the solid to the pre-weighed watch
glass labeled “ACE” and set it aside.
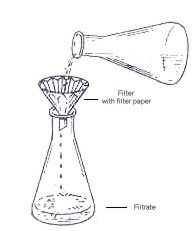
Figure 14: Gravity filtration |
Figure 15: Fisher-Johns Melting Point Apparatus |
Repeat the above procedure for the CAF flask. Add ~200mg of MgSO4
to the flask and swirl the flask to allow the MgSO4 drying
agent
to absorb any water in the mixture. Use gravity filtration to
remove
the drying agent from the flask, as depicted in Figure 3.14.
Label
a clean and dry 50 ml Erlenmeyer flask “CAF” and clamp the neck to a
ring
stand. Insert a glass funnel, equipped with filter paper into the
neck of the flask. Swirl the flask containing the CAF solution
and
the MgSO4 and quickly pour the mixture through the
filtration
apparatus to collect the drying agent in the filter paper. Place
the flask labeled “CAF” (contains the filtrate or the solution of CAF
in
CH2Cl2) on a hot plate stirrer. Add a
magnetic
stir bar to the flask, begin stirring and gently warm the mixture to
remove
all but ~5 ml of the CH2Cl2. Remove the
flask
from the heat, remove the stir bar and set the flask aside. Allow
the flask to cool down. The remaining CH2Cl2
should evaporate leaving a solid residue that is caffeine (CAF).
Weigh each of the flasks containing ASA and CAFand record the weights. Transfer the ASA and CAF from the 50 ml flasks to two small vials labeled with your name and ASA on one, and your name and CAF on the other. Wash and thoroughly dry the two flasks in the oven (~5-10min) Weign each clean, dry flask and substract this weight from the weight of the flask + ASA or flask + CAF to determine the masses of the recovered analgesics. Record the weights in the results section of your notebook.
Calculate
the percent recoveries of each analgesic from the tablets and
record
the percent recovery, and your calculations in your notebook.