
ACP Home | Organic Chemistry I | Organic Chemistry II | Lecture | Laboratory | General Chemistry
Organic Chemistry Laboratory
Simple and Fractional Distillation

ACP Home | Organic Chemistry I | Organic Chemistry II | Lecture | Laboratory | General Chemistry
Organic Chemistry Laboratory
Simple and Fractional Distillation
| A simple distillation apparatus is shown in Figure 1.2 below. Distillation involves selectively volatilizing (converting from the liquid phase to the gas phase) a component in a mixture. When a compound in the distilling flask is heated to its boiling point temperature, a phase change from the liquid state to the gas state is induced. The compound, in the gas phase, moves out of the distilling flask up into the other parts of the distilling apparatus, leaving behind the less volatile (higher boiling) components. When the gas vapors encounter the cold condenser tube (below the boiling point temperature) of the distilling apparatus, the gaseous compound reverts back to the liquid phase and drips into the collection flask, effectively separating the compound from the mixture. A simple distillation apparatus is depicted in Figure 1.2. To set up the distillation apparatus, set a stirrer/hot plate and ring stand in the hood. Place a 50ml heating mantle on the stirrer. Insert a 50ml round bottom flask (distilling flask) into the heating mantle and clamp the neck of the flask to the ring stand. Be sure to position the flask over the center of the stirrer plate. Measure out exactly 20 ml of commercial mouthwash using an Eppendorf pipe and weigh it. Record the weight. Dispense the liquid to be distilled into the distilling flask. Add a magnetic stir bar to the flask and continue to set up the simple distillation apparatus as shown in Figure 2. Attach a distilling head, thermometer adapter and thermometer. Position the thermometer bulb just below the “Y” of the distilling head. Place a second clamp on the apparatus at the joint between the distilling head and the thermometer adapter. (Never clamp anywhere except at the joints! It will crack the glassware.) Set up a second ring stand. Attach a condenserand vacuum adapter using Keck clamps. Attach a 25 ml round-bottomed collection flask and place a third clamp (clamped to a second ring stand)at the joint between the vacuum adapter and the collection flask. Be sure all the joints fit snuggly together, otherwise the apparatus will leak and reduce the efficiency of the distillation. Connect the water hoses to the condenser, with water “in” at the bottom, and draining “out” to the sink at the top end of the condenser. Connect the heating mantle to the Variac, and set the Variac at approximately 50V. Check your apparatus against the diagram in Figure 1.2, and the set-up in the lab before you continue. | 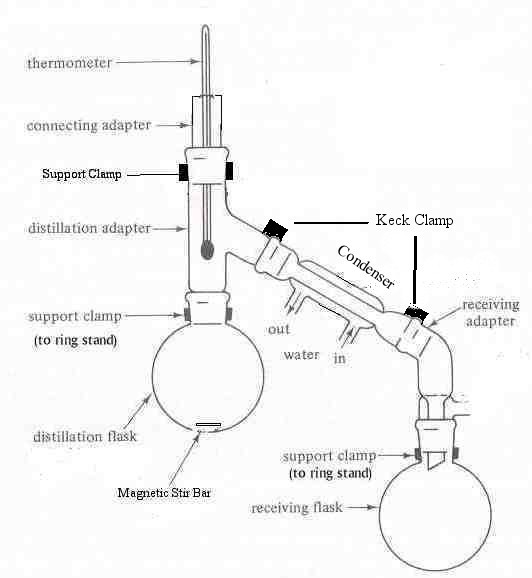 Figure 1.2: Simple Distillation Apparatus |