ACP
Home | Organic
Chemistry I | Organic
Chemistry II | Lecture
| Laboratory
| General
Chemistry
Organic
Chemistry
Laboratory
Recrystallization
and Drying Agents
Recrystallization
The principle of recrystalllization
is to dissolve the compound to be
purified in a warm solvent while the impurities either do not dissolve,
or the impurities remain dissolved after the pure compound crystallizes
from the solution when the solution is cooled back to room temperature
or put in an ice bath. A proper solvent must be selected. The
properties of a good recystallization solvent is one that the compound
dissolves easily when the solvent is warm, but that is less soluble at
room temperature or when cooled in an ice bath. The solvent
should relatively low boiling (typically below 100C). For
most organic compounds, water is not a good recrystallization solvent.
Recrystallization
requires significant patience so be prepared to be patient.
Procedure
Identify an appropriate solvent
to do the
recrystallization. Use the data generated from your solubility
experiments to select a proper recrystallization solvent for your
unknown. Do not use water or any of the other acidic or basic
aqueous solutions for recrystallization. For approximately 2
grams of a solid, use approximately 30-40 ml of recrystallization
solvent. Dispense the solvent into a 100ml beaker and
gently warm the solvent in a water bath to just below its boiling
point. Add the unknown to the warm solvent and swirl or stir the
solution. (If the compound does not completely dissolve, try adding
~5-10 ml more of solvent)
 Figure 1: Gravity Filtration
Figure 1: Gravity Filtration
|
If you observe particles in the
solution still, use a gravity filter
remove them (hot gravity filtration). Keep the solution warm and
set
up a clean
Erlenmeyer flask (125ml) with a glass funnel and piece of filter paper
(Figure 1 at left) and quickly pour the warm solution through
the filter. Set the the Erlenmeyer flask down and let is sit
undisturbed for 15-30 minutes, or until crystals or a precipitate
forms. If no precipitate or crytallization results after 30 minutes,
immerse
the beakier in an ice bath and allow it to cool down, undisturbed for
15-20 minutes.
Crystals (i.e., the pure compound) should form in the flask and leave
the soluble impurities behind in the solution. The pure compound
should be isolated using vaccum filtration (Figure 2 at right).
(DO NOT ATTEMPT TO FILTER IF THERE DOES NOT APPER TO BE ABOUT 1.5-1.8 g
of crystals formed). Collect the pure compound from the filter
paper and allow the material to dry for at least 24 hours before
attempting to take a melting point.
|
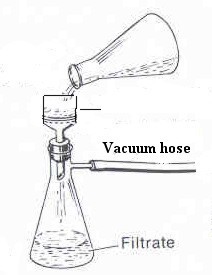
Figure 2: Vacuum Filtration
|
Drying Organic Solutions
The
process of isolation of pure organic compounds often requires removing
trace amounts of water. For example, in aqueous extractions trace
amounts of water will be transferred
into the organic layer because of the partial solubility of water in
the
organic phase. This water must be removed before the organic compound
can be properly characterized. Two general methods
of drying organic solutions are commonly used; saturated aqueous sodium
chloride and solid drying agents.
Saturated
Aqueous Sodium Chloride
The
bulk of the water can often be removed by shaking or "washing" the
organic layer with saturated aqueous sodium chloride. The salt water
(brine) works to pull the water from the organic layer to the water
layer. The concentrated salt solution tendency is to become more dilute
which promotes the migration of water from the organic layer into the
water layer.
Procedure
Place
the organic solution in a separatory funnel. The organic solvent might
be methylene chloride, diethyl ether, hexanes, etc., as long as it is
not, of course, water. Add an amount of saturated aqueous sodium
chloride, less than or equal to the amount of organic solution you
have.Stopper the funnel and shake as in an extraction. Allow the
layers to separate. The rules as to which layer is on top are the same
as for extraction. Drain
off the lower layer. In this case, this is the organic layer and the
layer you want to save. Dispose of the aqueous layer in the aqueous
waste carboy. (In some cases, for instance if you are using diethyl
ether, the upper layer will be the desired organic layer.)
Solid Drying
Agents
Final
traces of water are removed by treating the organic solution with a
drying agent. A drying agent is an inorganic salt which readily takes
up water to become hydrated. Several such salts are used routinely.
| drying agent |
capacity |
speed |
applications |
| calcium chloride, CaCl2 |
high |
medium |
used for hydrocarbons |
| calcium sulfate, CaSO4 (Drierite) |
low |
fast |
generally useful |
| magnesium sulfate, MgSO4 |
high |
fast |
not used for very acid-sensitive compounds |
| potassium carbonate, K2CO3 |
medium |
medium |
not for acidic compounds |
| sodium sulfate, Na2SO4 |
high |
slow |
generally useful |
Of
the five drying agents in the above table, magnesium sulfate is a fine
powder and the rest are of a larger particle size. Calcium chloride,
magnesium sulfate, and sodium sulfate are the three most commonly used
agents. Usually, a wash with saturated sodium chloride
solution to remove the bulk of the water is done before treating with a
solid drying agent.
Procedure
Add a small amount of the solid drying agent directly to the organic
solution. Swirl the solution. Observe the drying
agent; if it is all clumped together, add more. Click on the picture
link at the right to see how a solution with drying agent looks when it
is clumped and when it is free-flowing. Continue
swirling and observing the solution for 5-15 minutes, adding more
drying agent only until a fresh addition no longer forms clumps
Each
drying agent will have a slightly different appearance when “clumped”
and practice will make you better at judging whether or not the
inorganic salt is wet or dry. There is no set “rule” as to how much
drying agent needs to be added. The amount required depends on the
amount of water in the solvent solution which you are drying, and this
amount varies from experiment to experiment. Use as much as it takes to
dry the solution. In most cases, drying is as complete as it will get
in 20 minutes. When drying is complete, you need to remove the dried
organic solution from the drying agent. Usually gravity filtration is
used to remove the drying agent or decanting off the dried solution.


 Figure 1: Gravity Filtration
Figure 1: Gravity Filtration
