
ACP Home | Organic Chemistry I | Organic Chemistry II | Lecture | Laboratory | General Chemistry
Organic Chemistry Laboratory
Reflux

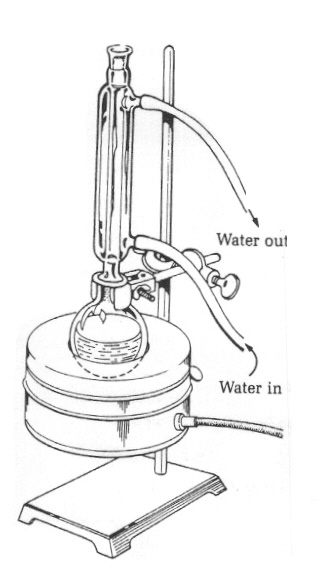
A simple reflux apparatus is shown on the right. To set up the reflux apparatus, set a stirrer/hot plate and ring stand in the hood. Place a heating mantle on the stirrer. Insert a round bottom flask (distilling flask) into the heating mantle and clamp the neck of the flask to the ring stand. Be sure to position the flask over the center of the stirrer plate. Measure out the reactants and solvent that will be used in the reaction and add them to the reaction flask, using a funnel. Add a magnetic stir bar to the flask and continue to set up the reflux apparatus as shown in Figure 1. Insert a condenser tube into the flask and clamp at the top joint of the condenser, if necessary, to stabilize the apparatus. Connect the water hoses to the condenser, with water “in” at the bottom, and draining “out” to the sink at the top end of the condenser. Connect the heating mantle to the Variac, and set the Variac at approximately 50V. Check your apparatus against the diagram in Figure 1. Carefully turn the water on, turn on the magnetic stirrer and the Variac (heat). As the apparatus is heated, the liquid in the reaction flask will begin to bubble. The actual refluxing will begin when the solvent volatilizes and moves up into the condenser tube where it experiences a lower temperature. As the vapor rises through the condenser (which is cold from the water running through it) the solvent will condense back to the liquid phase and drip into the reaction flask. |
|
 |