
Organic Chemistry Laboratory I
Spectroscopy
Experiment: Infrared, Nuclear Magnetic
Resonance and Mass
Spectroscopy
Experiment Procedure

Organic Chemistry Laboratory I
Spectroscopy
Experiment: Infrared, Nuclear Magnetic
Resonance and Mass
Spectroscopy
Experiment Procedure
| Spectral
Data Master Unkown List (pdf) |
|
| 1-50 |
101-150 |
| 51-100 | 151-200 |
| 201-250 | |
| Spectroscopy
Workshop Schedule |
|
| Monday
11/26
9:30-11:00am OB110 |
Monday
11/26
4:00-5:30pm OB218 |
| Tuesday
11/27
11:00-12:30pm OB102A |
Wednesday
11/28
12:30-2:00pm OB110 |
| Thursday
11/29
4:30-6:00pm CL208 |
Friday
11/30
9:30-11:00am
OB110 |
| Step 1 | Identify all the major peaks in the spectrum between 3600cm-1 and 1500cm-1 (+/- 100cm-1). Label the peaks on the spectrum as 1,2,3 etc and prepare a table in your notebook with four columns with the following headings: Peak Number, Absorbance Range, Absorbance Intensity, Bond Type. Label the Table as Analysis of IR Spectrum for Unknown # ___. Enter all peak numbers in the "Peak Number" column, their corresponding absorbance range in the "Absorbance Range column, and the absorbance intensity (Strong =50% of scale or more; Medium 25-505 of scale; Weak = less than 25% of scale) in the "Absorbance Intensity" column. |
Step 2 |
Is there a peak between 1650-1850cm-1 and 3600-3200cm-1? If yes go to Step 3. If no go to Step 5. |
| Step 3 | Is the peak between 3600-3200cm-1 broad and
intense?
If no, go to Step 4. If yes, the compound probably contains one
of the following functional groups or functional group combinations. 1) Carboxylic acid 2) Ketone and alcohol or phenol 3) Aldehyde and alcohol or phenol 4) Ester and alcohol or phenol 4) Alkene and Alcohol or phenol 5) Phenol List these possible functional groups in the table under the "Bond Type" column next their corresponding peaks listed in the table. |
| Step 4 | Is the peak between 3600-3200cm-1 sharp and
medium to weak
in intensity? If yes, the compound probably contains one of
the
following functional groups or functional group combinations: 1) Secondary amide (if only one peak between 3600-3200cm-1) primary amide (if two peaks between 3600-3200cm-1) or 2) Ketone and amine or 3) Aldehyde and Amine List these possible functional groups in the table under the "Bond Type" column next their corresponding peaks listed in the table. |
| Step 5 | Is there a peak between 1650-1850cm-1 ? If
no, go
to Step 6. If yes, the compound contains one of the following
carbonyl
or C=C containing functional groups: 1) ester 2) ketone 3) aldehyde 4) tertiary amide 5) alkene (~1660cm-1) 6) aromatic (~1450 and 1600cm-1) List these possible functional groups in the table under the "Bond Type" column next their corresponding peaks listed in the table. |
| Step 6 | Is there a peak between 3600-3200cm-1
? If no,
go to Step 7. If yes, the compound contains one of the following
functional groups: 1) Alcohol (if the peak is broad and strong in intensity) 2) Amine (if the peak is sharp and medium in intensity) List these possible functional groups in the table under the "Bond Type" column next their corresponding peaks listed in the table. |
| Step 7 | The compound contains one or more of the following functional
groups: 1) Alkane 2) Ether 3) Alkyl Halide List these possible functional groups in the table under the "Bond Type" column next their corresponding peaks listed in the table. |
Step 8 |
Review the list of bond
type/functional groups in the table you completed. Cross out all
compounds on the master unknown compound list that contain functional
groups that are not on your list.
Proceed to the crude analysis of the NMR spectrum of your unknown. |
Step 1 |
Identify all the peaks in the
NMR spectrum. Label the peaks in the spectrum as 1,2,3 etc and
prepare a table in your notebook with four columns with the following
headings:
Peak Number, Chemical Shift, Multiplicity, and Proton Type.
Label the Table as Analysis of NMR
Spectrum for Unknown # ___. Enter
all peak numbers in the "Peak Number" column, their corresponding exact
chemical shift range in the "Chemical Shift" column, and the
multiplicity (singlet, doublet, etc..) in the "multiplicity"
column. Use the information in Table 5 of the background
reading to identify the specific proton type. Fill this
information into the table under "proton type" for each peak. |
Step 2 |
Are there any peaks in the range
of 6.5-8.0 ppm? (aromatic region) If yes, your compound
contains aromatic protons, likely a benzene or related aromatic ring.
Eliminate all remaining compounds on the master unknown compound
list that do not contain an aromatic ring. If no, your compound
does not contain a benzene or related aromatic ringEliminate all
compounds on the list that do contain an aromatic ring. |
Step 3 |
Are there any peaks in the
region between 10-12ppm? If yes, your compound contains a
carboxylic acid or aldehyde. Eliminate any remaining compounds on
the master unknown compound list that do not contain one of these
functional groups. If no, your compound does not contain an
aldehyde or carboxylic acid. Eliminate any remaining compounds on
the master unknown compound list that do contain one of these
functional groups. |
| Step
1 |
Prepare a section in your
notebook for recording data from the mass spectral analysis.
Label the section in the notebook as Mass Spectral Analysis of Unknown # ___ |
| Step
2 |
Identify the parent peak (M+)
in the spectrum and record the m/z value for the parent
peak. This m/z value corresponds to the molecular weight of
the
unknown compound. |
| Step
3 |
Look for M++2 peaks that are at least 20-25% relative abundance (intensity). No M++2 peak indicates that there is no chlorine or bromine atom in the unknown compound. The presence of an M++2 peak that is ~25% the relative intensity of the M+ peak indicates that the unknown contains a chlorine atom. If the compound contains a chlorine, review the remaining compounds on the list and eliminate any compounds that do not contain a chlorine. If the compound does not contain a chlorine, eliminate any compoundson the list that do contain a chlorine. |
| Step
4 |
The presence of an M++2
peak that is ~50% the relative intensity of the M+
peak indicates that the unknown contains a bromine atom. If
the compound contains a bromine, eliminate any compounds remaining on
the list that do not contain a bromine. If the compound does not
contain a bromine, eliminate any compounds from the list that do
contain a bromine atom. |
Step 5 |
Review the compounds on the list
and using Cherm finder or another resource, find the molecular weights
of the remaining compounds on the list. Eliminate any compounds
on the list that do not have molecular weights consistent the m/z value
of the parent peak in the spectrum. |
| Step
1 |
Set up a new section in the notebook
labeled Detailed Ananlysis of the NMR Spectrum of Unknown # ___.
Draw the
structures of all of the remaining compounds, drawing out all of the
hydrogen atoms in each structure. |
| Step
2 |
In your
notebook, set up a
table for each structure that you drew that lists the different types
of protons in the structure, the predicted chemical shift of each
proton or group of protons, and the predicted multiplicity of each
proton/group of protons. |
| Step
3 |
In your
notebook, sketch a
predicted NMR spectrum from the data compiled in each table for each
remaining compound. |
| Step 4 |
If the sketched spectrum contains extra peaks, peaks with multiplicities different from the provided spectrum or has peaks missing, it is not consistent with the unknown spectrum. Compounds whose sketched spectrum is not consistent with the provided spectrum can be eliminated as possibilities. |
| Step 5 |
The compound whose sketched
spectrum correlates the best
with the provided spectrum and is completely
consistent with the provided spectrum corresponds to the
identity of the unknown. |
| Compounds |
Proton
Types |
Sketched
Spectra |
|||||||||||||||
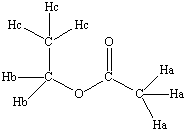 ethyl acetate |
|
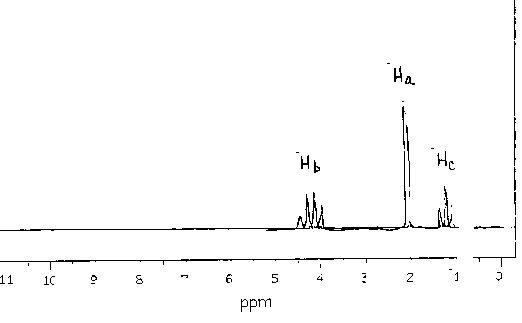 |
|||||||||||||||
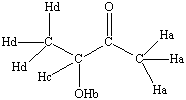 3-hydroxy-2-butanone |
|
 |
|||||||||||||||
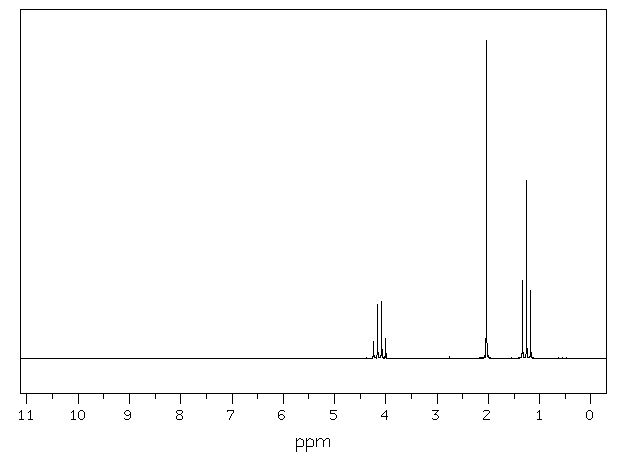 Provided Spectrum |
|||||||||||||||||