Organic Chemistry Laboratory II
Synthesis of Banana Oil
(Esterification)
Experiment Background

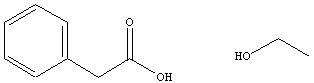
Esters can be prepared through a variety of methods, but the most
common
is the Fisher esterification method. The Fisher esterification is
an acid catalyzed reaction that is used to generate esters from
carboxylic
acids and alcohols. The appropriate starting materials for
preparing
a specific ester can be determined by examining the structure of the
ester.
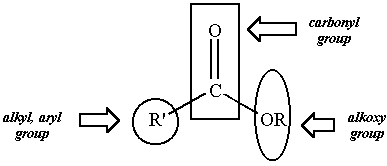
The carboxylic acid starting material can be revealed by identifying
the
carbonyl group and the alkyl or aryl substituent directly bonded to
that
carbonyl group. Attachment of a hydroxyl group (-OH) to the
carbonyl
carbon provides the appropriate carboxylic acid starting
material.
The alkoxy group bonded to the carbonyl carbon of the ester is derived
from the alcohol starting material. Replacing the carbonyl carbon
of the ester with a hydrogen atom (-H). Some esters and the
carboxylic
acids and alcohols from which they are derived are given in table
below.
|
|
|
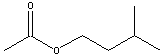 |
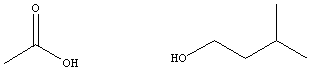 |
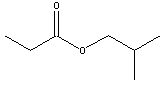 |
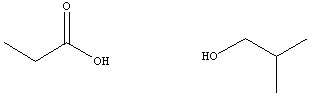 |
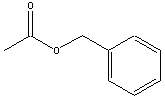 |
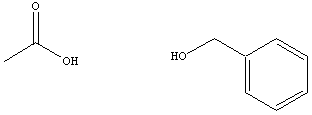 |
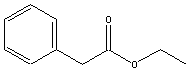 |
 |
The Fisher Esterification
The Fisher esterification is a classic organic reaction that involves
the acid-catalyzed conversion of a carboxylic acid and alcohol to an
ester
functional group via a nucleophilic acyl substitution. The Fisher
esterification is a reverible
reaction
and often requires special reaction conditions to promote the formation
of the ester product.
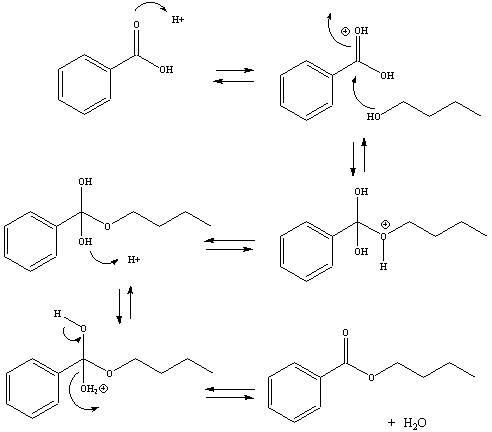
Mechanism of the Fisher Esterification
The Fisher esterification is a nucleophilic acyl substitution
reaction.
Under acidic conditions, the carbonyl-group of the carboxylic acid
becomes
protonated. This makes the carbonyl-carbon more electrophilic and
reactive with the nucleophilic alcohol oxygen. The oxygen atom of
the alcohol forms a bond with the carbonyl carbon, forcing the
carbon-oxygen
pi bond to break. Through a series of additional steps (outlined
below) the hydroxyl group of the carboxylic acid is eventually lost to
generate the ester, the substituted product and water.
Fisher Esterification Equilibria
Each step of the Fisher esterification is reversible at temperatures
that are typically used to run the reaction. The equilibrium
constant
for the reaction between acetic acid and isopentyl alcohol to
generate
isopentyl acetate and water is ~4, and is represented by the
expression:
Keq =
[isopentyl
acetate] [H2O]
[acetic acid] [isopentyl alcohol]
The reaction is run under thermodynamic conditions, where
equilibrium
is achieved. La Chatelier’s principle predicts that the equilibrium of
a chemical reaction will be restored (i.e, the Keq at a given
temperature
will be maintained) even when concentrations of reactants or products
are
altered throughout the reaction. The theoretical yield of the
ester
product in the Fisher esterification, when equimolar quantities of
acetic
acid and isopentyl alcohol are used is only ~55%. The yield of
product
can be enhanced by increasing the concentration of either the acid or
the
alcohol starting materials. In this reaction, there is
approximately
3 times more acetic acid than alcohol. The higher concentration
of
acid increases the theoretical yield of the reaction. While this
strategy improves the yield of the reaction product, a consequence is
that
the product will be contaminated with starting material.
Therefore,
the crude reaction mixture must be purified first by washing in the
separatory
funnel, then by distillation.