Organic
Chemistry II | Lecture
| Laboratory
Organic Chemistry Laboratory II
The Diels Alder
Reaction: Reaction of 2,4-Hexadien-1-ol
with Maleic Anhydride
Experimental Procedure
|
In the hood, fill a crystallization dish
about 1/2 to 3/4 with water and set it on a
stirrer/hot plate. Tare a 50 ml Erlenmyer flask and weigh out
2,4-hexadien-1-ol (0.40g)
into the flask. (Note: This may be
challenging as the compound is low melting, meaning is
may start out as a liquid but solidify as you work
with it.). Add
toluene (5ml) to the flask using a glass pipet,
rinsing down any of the compounds that may have
adhered to the side of the flask. Use a piece of
weigh paper to weigh out maleic anhydride (0.40g) and
transfer to the flask.
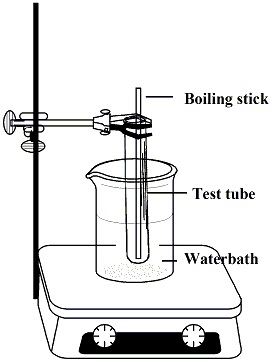
Clamp the flask so that it is emersed in the water bath.. |
Figure 1: Reaction Set up |
|
|
|
Once cooled, empty the hot water from the
crystallization dish and replace it with ice. Emerse the clamped flask
containing the reaction mixture into the ice bath and
allow it to sit for ~10 minutes to ensure complete
crystallization of the product occurs. While the
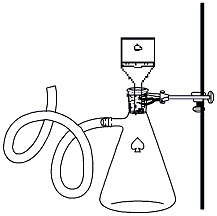
reaction is cooling, set up an apparatus for vacuum
filtration. |
|
Figure 3: Vacuum filtration |
While the product is drying in the hood, run an IR spectrum of
the two starting materials. Students may work in groups
of four to run these spectra. Each bench should run one
spectrum of the 2,4-hexadien-1-ol
and one spectrum of maleic anhydride. For infromation regarding IR spectroscopy
click here.
Label a small vial with your name, date, lab section, and
Diels-Alder product. Transfer the product from the watchglass to the vial. Hand in
your product to the lab instructors for storage until week 2.
Waste Disposal (Waste containers located in the
back hood.)
Dispose of all non-halogenated organic compounds/solvents in “non-halogentaed
organic waste”. Glass microcapillary
pipets and pipets should be disposed of in designated
containers. Filter paper is disposed of in the solid
waste
End of Week 1
Collect
your
product vial from the previous week
from your lab instructor. Weigh the dried
product, record the mass and calculate the percent
yield. Determine the melting point of your reaction
product and record this value along with the literature
melting point value of the product in your notebook. Run
an IR spectrum of your product. (Each individual student must
run their own spectrum). Compare the IR spectrum of your
product with the spectra of the starting materials.
Tabulate the major peaks in each spectrum and the
corresponding bond/functional groups in the structures that
give rise to these peaks. Print a copy of the proton
NMR
spectrum of the product.
End of Week 2