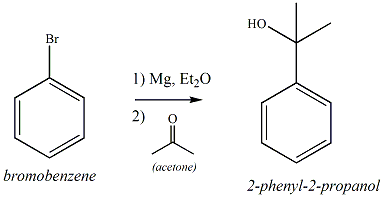
Organic Chemistry Laboratory II
Preparation of 2-Phenyl-2-propanol (Grignard
Reaction)
Experiment Description

Preparation of the Grignard Reagent
Add 2g of magnesium turnings and a magnetic stir bar to a dry 100 ml
round-bottomed flask, and place the flask in the drying oven for 30
minutes.
Remove the flask from the oven, clamp it to a ring stand, fit the
flask into a heating mantle and immediately attach a drying tube.
Position the flask on a stirrer/hotplate. Set the reaction up on
the side of the hood near to the faucet labeled for distillation.
Allow the reaction flask to cool to room temperature completely before
proceeding. While the flask is cooling, place a 150ml beaker into
the drying oven.
Once the flask has completely cooled to room temperature, remove the drying tube and add 15 ml of anhydrous diethylether and 6 ml of bromobenzene using a glass funnel. If the flask is not sufficiently cool, the ether will evaporate immediately. If it evaporates, add more ether. Re-insert the drying tube into the neck of the flask and turn on the magnetic stirrer. If the reaction does not begin immediately, carefully use a dry stirring rod to crush one of the magnesium turnings against the bottom of the flask. Be careful not to press too hard and crack the flask! Alternatively, a single crystal of iodine can be added to help initiate the reaction. A cloudy solution is an indication that the reaction has begun. Once the reaction has started, attach a dry condenser tube, and add an additional 25 ml of ether through the top of the tube using a glass funnel. Connect the drying tube to the top if the condenser. Connect the water hoses to the condenser and turn the water on. Use a permanent marker to mark the level of the contents of the mixture. If the reaction becomes too vigorous, cool the flask for 2-3 minutes in an ice water bath.
Once the reaction has become steady, warm the flask in the heating
mantle
on low heat for 5-10 minutes until the magnesium has completely
dissolved/reacted
and the solution has a brown or cloudy appearance. If the mixture has
evaporated
more than 0.5 cm below the starting line, add an additional 5ml of the
ether. If loss of ether continues, turn off the heat or immerse
the
flask into an ice bath to cool it down. Allow the reaction
mixture
to cool to room temperature (if hot). Remove the condenser tube
and
replace it with the drying tube. Set the flask aside, keeping it
clamped securely to the ring stand. This Grignard reagent will
decompose
rapidly, so the next step must be started at once.
For the final step of the reaction, add 20 ml of 3M HCl to the
flask.
This will hydrolyze the alkoxy/magnesium bromide salt to form the
2-pehnyl-2-propanol. Transfer the reaction mixture to a 200ml
beaker. Add an additional 20 ml of ether to the rb flask to
remove any residual product and add it to the beaker. Two layers
should be apparent in the beaker and no solids should remain in the
reaction flask.
If solid still remains, add more ether (~10ml). Transfer the
entire
reaction mixture to a 125ml separatory funnel (Be sure the stopcock is
in the closed position). Two layers should appear. The top
layer is the ether layer, containing product. The bottom
layer
is the aqueous layer containing the magnesium bromide salt
by-product.
Label two 100ml beakers or Erlenmeyer flasks as “water layer” and
“organic
layer". Drain the lower layer from the separatory funnel into the
beaker or flask labeled “water layer”. Add 10ml of deionized
water
to the separatory funnel. Stopper the funnel and shake vigorously
for 1-2 minutes. Set the separatory funnel back onto the ring
stand
and allow the two layers to separate. Drain the bottom layer into
the beaker or flask labeled “water layer”. Add 10ml more of the
deionized
water to the separatory funnel and repeat the process one last time,
combining
the lower layer again into the “water layer”. Pour the remaining ether
layer into the dry 100 ml beaker labeled "organic layer". Add ~100mg of
magnesium sulfate drying agent to the beaker or flask labeled “organic
layer”. Swirl the flask, then allow the drying agent to
settle
to the bottom. Carefully decant the solution into a pre-weighed,
clean, very dry 100 ml beaker, leaving the drying agent behind. Place
the
beaker containing the ether layer in a warm water bath and heat to
remove
the ether. Store your product in an open beaker to allow it
to finish drying.
IR Spectroscopic Analysis of 2-Phenyl-2-propanol
Prepare a sample of the product for IR analysis. Run a background
scan if
one
has not yet been for the lab, and then record a spectrum of the
product. Set up a table in your notebook and record the major peaks (
and frequencies in cm-1) in the table.
TLC Analysis of 2-phenyl-2-propanol
Prepare a TLC plate with two tick marks (labeled 1 and 2) to
analyze your synthesized product (1) and
benzene (2).
Use the EtOH solution from the vial labeled "benzene" for
the TLC analysis. Prepare a solution of your
product
in ethanol (10mg product/1-2ml EtOH). Spot the plate with the
solutions
using two capillary pipettes and allow the spots to dry completely
before
developing the plate. Develop the plate using a developing
solvent
that will provide an Rf of ~0.5. Start with 25:75 ethyl acetate:
hexane
and make appropriate adjustments in the developing solvent ratio, if
necessary.
View the plate under UV light and in the iodine chamber.
Calculate
Rf values for all the spots observed. What do the relative Rf
values tell you about the success of the reaction and the purity of the
product?