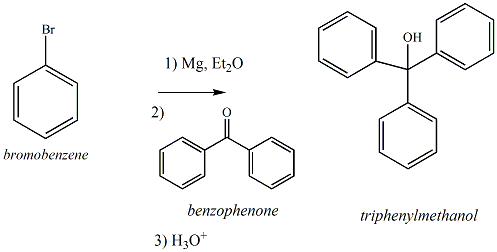
Organic Chemistry Laboratory II
Preparation of Triphenylmethanol (Grignard
Reaction)
Experiment Procedure

Preparation of the Grignard Reagent
Rinse a 10ml graduated cylinder, a stir bar and a condenser tube
with acetone. Allow the acetone to fully
evaporate. Retrieve a 100ml round-bottomed flask from the
drying oven and immdediately attach a drying tube to the top of
the flask. When cool enough to handle, clamp the flask to a
ring stand in the hood, positioning the flask in a heating mantle
set on a stirrer/hotplate. Set the reaction up on the side of the
hood near to the faucet labeled for distillation.Retrieve a vial
containing ~0.5g of pre-weighed magnesium turnings from the drying
oven and add the turnings to the clamped flask, immediately
returning the drying tube to the top of the flask. Allow the
flask to cool completely to room temperature. Measure
out 2.3ml of bromobenzene, using the 10ml graduated cylinder and
add the bromobenzene to the 100ml rb flask. Using the same
graduated cylinder (no need to rinse or clean it), measure 5 ml of
diethylether and add it to the rb flask. If the flask is not
sufficiently cool, the ether will evaporate immediately. If
it evaporates, add more ether. Re-insert the drying tube and
continue to stir the reaction. Add the stir bar to the
flask, replace the the drying tube and begin stirring the
reaction. Wait for the reaction to start ( solution will become
cloudy and yellowish).
|
If the reaction does not
begin immediately, carefully use a dry stirring rod to
crush one of the magnesium turnings against the bottom of
the flask. Be careful
not to press too hard and crack the flask!
A cloudy solution is an indication that the reaction has
begun. Once the reaction has started, add an
additional 10 ml of ether using a glass funnel.
Remove the drying tube and attach a reflux condenser to
the flask. Insert the drying tube on the top of the
reflux condenser. Connect the water hoses to the
condenser and turn the water on. Use a permanent
marker to mark the level of the contents of the mixture.
If the reaction becomes too vigorous, cool the flask for
2-3 minutes in an ice water bath.
Once the reaction has
become steady, warm the flask in the heating mantle on low
heat for 10 minutes until the magnesium has completely
dissolved/reacted and the solution has a brown or cloudy
appearance. If the mixture has evaporated more than 0.5 cm
below the starting line, add an additional 5ml of the
ether. If loss of ether continues, turn off the heat
or immerse the flask into an ice bath to cool it
down. Allow the reaction mixture to cool to room
temperature (if hot). Set the flask aside,
keeping it clamped securely to the ring stand. This Grignard reagent will
decompose rapidly, so the next step must be started at
once.
Preparation of Triphenylmethanol Remove a dry 150 ml Erlenmyer flask from the drying oven and allow it to cool to room temperature. Place 3.7g of benzophenone into the beaker and add 10 ml of ether. Gently swirl the beaker to completely dissolve the benzophenone. When the reaction mixture if fully cooled to room temperature, remove the condenser tube and drying tube from the reaction flask containing the Grignard reagent, insert a funnel and begin stirring the solution. Add the benzophenone solution dropwise to the flask using a glass pipet (from the |
|
 |
When the reaction is cooled, remove
the drying tube and condenser. Continue stirring the mixture
and slowly add
5
ml of distilled water dropwise using a glass pipet. This will
hydrolyze the alkoxy/magnesium bromide salt to form the
triphenylmethanol. The water will also react with any
unreacted Grignard reagent to form benzene. After the addition of
the water is complete, 15ml of 5% HCl are added with continued
stirring. Continue stirring the reaction mixture until the
reaction subsides. Transfer the reaction mixture to a 200ml
beaker, adding additional ether (5-10ml) to the rb flask if
necessary to remove any residual product. Swirl the beaker
and add more ether (5-10ml) to dissolve any solids. Two
layers should be apparent in the beaker and no solids should
remain in the reaction flask.
 |
|
Transfer the entire reaction mixture (using a funnel) to
a 125ml separatory funnel (Be sure the stopcock is in the
closed position). Two layers should appear.
The top layer is the ether layer, containing
product. The bottom layer is the aqueous layer
containing the magnesium bromide salt by-product.
Label two 100ml beakers or Erlenmeyer flasks as “water
layer” and “ether layer". Drain the lower layer from
the separatory funnel into the beaker or flask labeled
“water layer”. Add 15 ml of aqueous 5% sodium bicarbonate to
the ether layer in the separatory funnel.
Stopper the funnel and shake vigorously for 1-2
minutes. Vent the funnel into the back of the
hood. Set the separatory funnel back onto the ring
stand and allow the two layers to separate and remove the
stopper. Drain the bottom (water) layer into the
beaker or flask labeled “water layer”. Add 15ml m of
saturated sodium chloride to the separatory funnel and
repeat the process above, combining the lower layer again
into the “water layer”. Pour the remaining ether layer
into the dry 100 ml beaker labeled "ether layer". Add
~100mg (tip of a spatula) of magnesium sulfate drying
agent to the beaker or flask labeled “ether
layer”. Swirl the flask, then allow the drying
agent to settle to the bottom. Decant or filter off
the magnesiun sulfate and transfer the solution into a
pre-weighed, clean, very dry 125 ml Erlenmeyer
flask. Clamp the flask containing the ether layer to a
ring stand and place it in a warm water bath to remove the
ether. Store your product in the open
Erlenmyer flask to allow it to finish drying over the
week. |
Weight and Melting Point Determination of
Triphenylmethanol
Inspect your product and if it appears to be dry, weigh it and
calculate the percent yield. Calibrate the thermometer
of the melting point apparatus with benzoic acid (lit mp = 122oC).
Record
the
melting point of the product. Transfer the product to a
clean vial labeled with your name, lab day, mass (in g) and
experimental melting point. Hand in the labeled vial
containing the product.
Waste Disposal
Dispose of all organic waste (ether, hexane, ethanol,
acetone) in the "non-halogenated" waste container. The
pH of aqueous solutions should be checked. Acidic
solutions should be disposed of in "acidic aqueous waste" and
basic solutions in "basic aqueous waste".
Used filter paper and TLC plates,MgSO4 and organic
solids should be disposed of in the solid waste containers.
Dispose of pipets, melting point cover slips, melting point
tubes in designated waste containers.