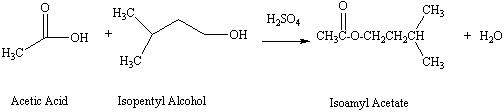
Organic Chemistry Laboratory II
Synthesis of Banana Oil
(Esterification)
Experimental Procedure

|
Figure 2: Using a powder funnel to add reagents to the flask |
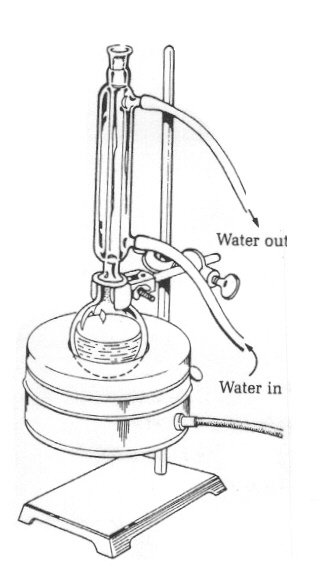 Figure 3: Reflux apparatus |
Plug the Variac into the wall outlet and set the dial to between 50 and 60V. Heat the reaction mixture to reflux (i.e, until the contents is boiling and the vapors condense and drip back into the flask) for one hour. While the mixture is heating, dispense 100ml of deionized water into a clean 200ml Erlenmeyer flask, clamp it to a second ring stand and cool it in an ice bath.
Crude Purification of the Reaction Mixture in a Separatory
Funnel
Allow the reaction mixture to cool to room temperature. Pour the
cooled
mixture into a 125 ml separatory funnel set in a ring, clamped to
a
ring
stand. Check that the stop cock on the funnel is in the closed
position
and add 55 ml of the cold deionized water to the funnel. Add
10ml
of the cold deionized water to the reaction flask, swirl and add
the
water
to the separatory funnel. Stopper the funnel, invert and
shake
the
mixture for 25-30 seconds. Dispense the lower (aqueous)
layer
into
a 200 ml beaker, labeled "aqueous layer". The organic layer
containing
the ester product will remain in the separatory funnel.
To remove any leftover acetic acid from the ester solution (organic layer), wash the organic layer by adding 25ml 5% aqueous sodium bicarbonate to the separatory funnel. Stopper the funnel, invert and shake for 25-20 seconds, being sure to vent the funnel frequently by opening the stopcock. Drain the lower, aqueous layer into the flask labeled “aqueous layer”. Wash again with a second 25 ml aliquot of 5% sodium bicarbonate. Check the pH of the “water layer” flask. If the solution is basic, move on to the next step. If the solution is acidic, wash one more time with the 5% sodium bicarbonate. Add 25ml of saturated NaCl solution to the separatory funnel. Stopper the funnel, invert and shake the mixture for 25-30 seconds. Dispense the lower (aqueous) layer into a 125 ml flask, labeled "aqueous layer". The organic layer containing the ester product will remain in the separatory funnel.
Drain the organic layer remaining in the funnel into a clean, dry 125 ml Erlenmeyer flask. Add ~2g MgSO4 to the flask and cover the flask with Parafilm. Swirl gently until the liquid is clear. If the cloudiness persists, add an additional 0.5g MgSO4. Decant the solution into a clean, dry Erlenmeyer flask, cover with Parafilm and keep the ester solution in the lab drawer until week 2.
IR Spectroscopic Analysis of Isopentyl Acetate
Place 2 drops of the isopentyl acetate product between two salt
plates
(Figure 5) and use the IR spectrophotometer to generate an IR
spectrum.
Place two drops of the known isoamyl acetate product between two
salt
plates
and use the IR spectrophotometer to generate an IR spectrum. Run a
background
scan if one has not yet been for the lab. Compare the spectrum of
the
experimental
product to the spectrum of the known isopentyl acetate.
Record
all
the major peaks in the spectrum.