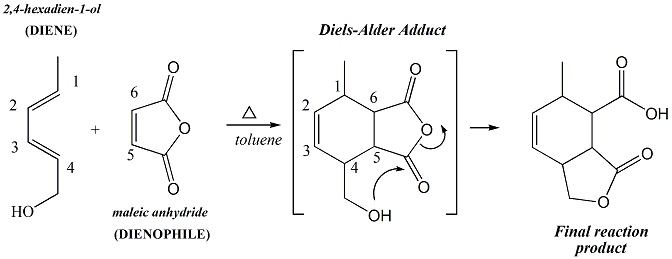
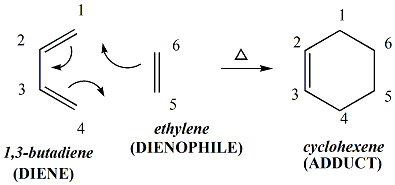
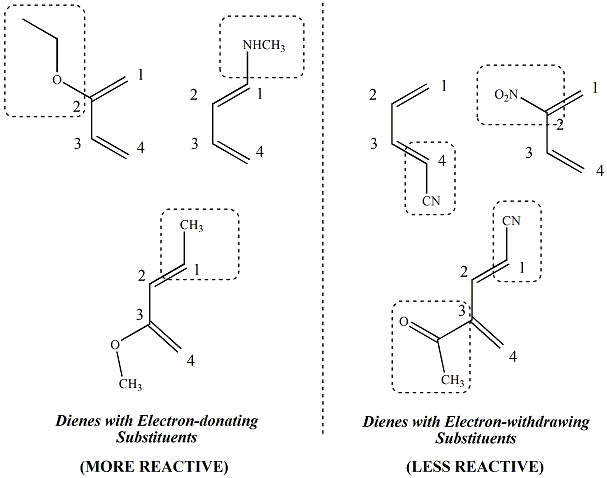
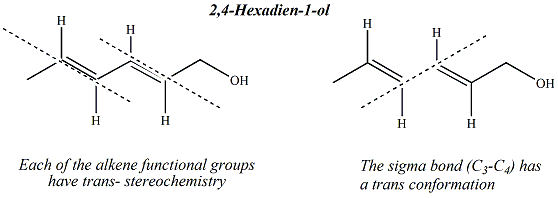
Spectroscopic
Characterization of Reaction Products
Infrared (IR) and proton nuclear magnetic
resonance (NMR) spectroscopy are complementary
and powerful analytical methods for elucidating
the structure of reaction products. IR
spectroscopy provides information about specific
functional group transformations that occur in
the reaction, while NMR spectroscopy allows for
the verification of the carbon skeleton in the
product. To learn more about IR and proton
NMR spectroscopy click on the link
here.
IR
Spectroscopy
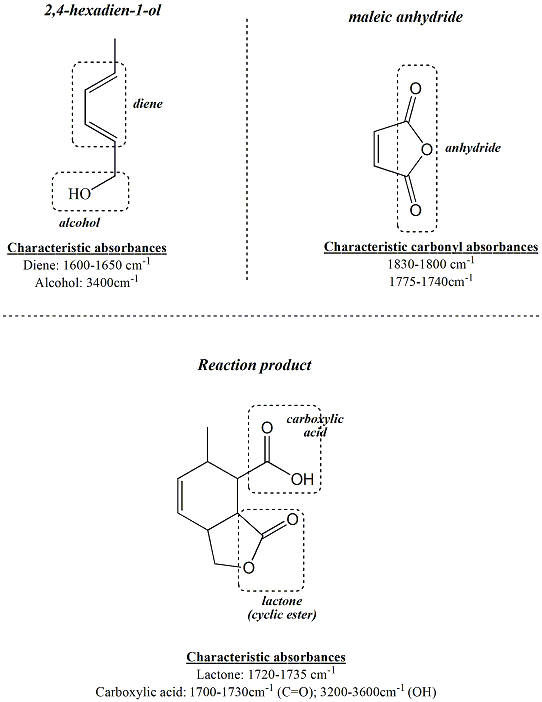
For the
reaction of 2,4-hexadien-1-ol with maleic
anhydride, IR spectroscopy helps to
demnostrate that the functional groups in the
starting materials have been converted to
different functional groups in the
product. 2,4-Hexadien-1-ol contains a
conjugated diene, thus it is anticipated that
the IR spectrum will contain peaks
corresponding to this conjugated functional
group. Likewise, maleic anhydride
contains anhydride carbonyl groups that have
characteristic absorptions in the IR spectrum,
one near 1830-1800 cm-1 and one
near
1775-1740 cm-1 and no absorbance in
the -OH region between 3200-3400 cm-1.
In the predicted product, there is an ester
which has a carbonyl absorbance in the
1720-1735cm-1 range, a carboxylic
acid, which has a carbonyl absorbance in the
1700-1730cm-1 range and a broad
stretch in the OH region between 3200-3600cm-1.
The differences between the product IR
spectrum and the starting materials, should
indicate the reaction occurred. However,
additional verification by NMR spectroscopy,
melting point determination and TLC analysis
are needed for further verification of the
reaction product.
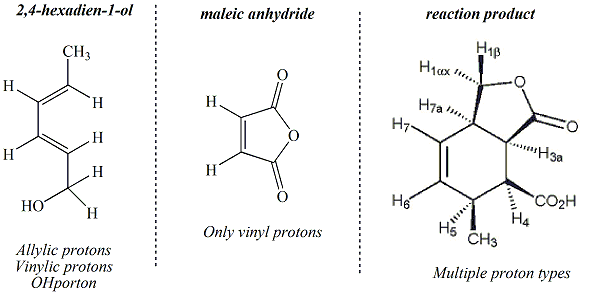
Only protons
(i.e., hydrogen atoms) in a compound give rise
to peaks in the NMR spectrum. Depending
on what other atoms that proton is bonded to,
it will have a specific chemical shift (i.e.,
ppm value) that is characteristic of that
proton type. FOr example, a hydrogen
atom directly bonded to a carbon of an alkene
or diene is referred to as vinylic and will
have a chemical shift between 4.5 and 6.5
ppm. Examination of the structure of the
starting materials and the reaction product,
specifically focussing on the hydrogen atoms
in these structures reveals that each
structure has a unique set of proton types
that will give rise to very different NMR
spectra. Each proton in the structure
must correspond to a peak in the NMR
spectrum. Maleic anhydride has a very
simple NMR spectrum since there is only one
type of proton (vinylic).
2,4-Hexadien-1-ol has a more complex spectrum,
with allylic, vinylic and OH protons.
The reaction product has many different proton
types and is predicted to have quite a complex
spectrum. Use the guidelines outlined at
this
link
to interpret the NMR spectrum of your reaction
product, matching each peak in the spectrum to
a specific proton in the structure.