Mechanism of the Friedel Crafts Reaction
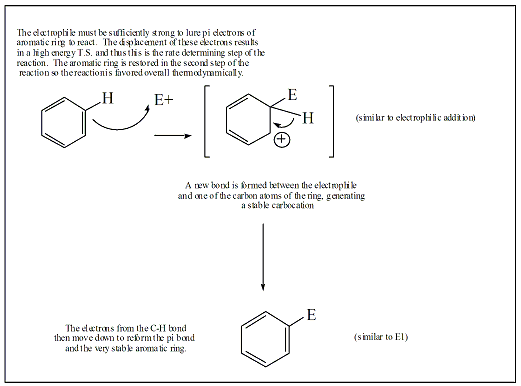
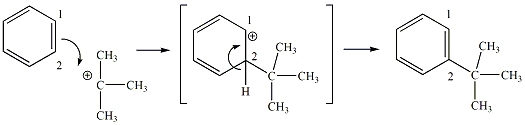
The Friedel-Crafts alkylation reaction is one of five types of electrophilic aromatic substitution (EAS) reactions. All five specific types of EAS follow the same reaction mechanism. The general mechanism of the EAS reaction is shown in Figure 2, and a specific mechanism with a tertiary carbocation is shown in Figure 2a. The pi electrons of one of the pi bonds (C1-C2) of benzene is used to form a new bond between C2 and the carbon atom of the carbocation. This results in the generation of a new carbocation intermediate, where the carbocation is localized on C1. The hydrogen at C2 gives up its electrons from the C2-H bond to regenerate the pi bond between C1 and C2, restoring the aromatic character to the benzene ring.

Figure
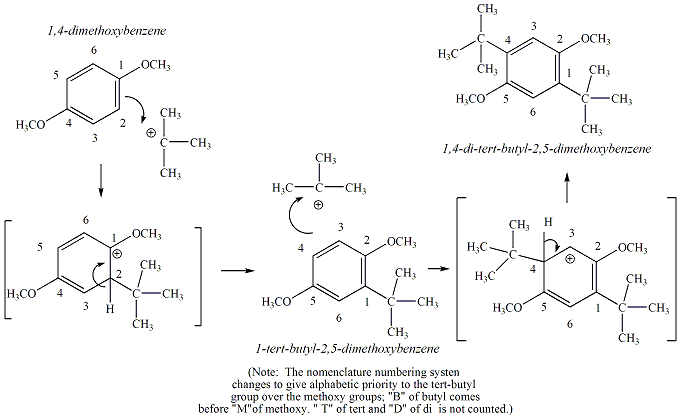
1: Reaction scheme depicting the Friedel Creafts reaction
of 1,4-dimethoxybenzene with tert-butyl alcohol