Synthesis and Analysis of Soap (Saponification)
Background Reading
Ester Hydrolysis
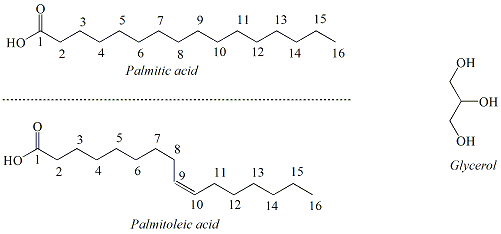
Figure 3
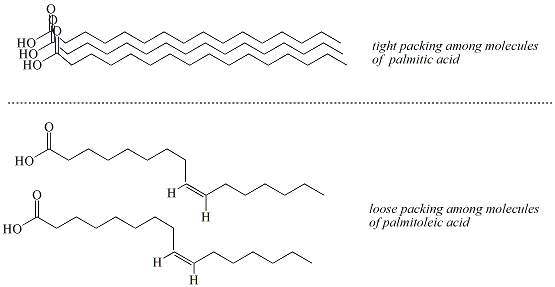
Figure 4
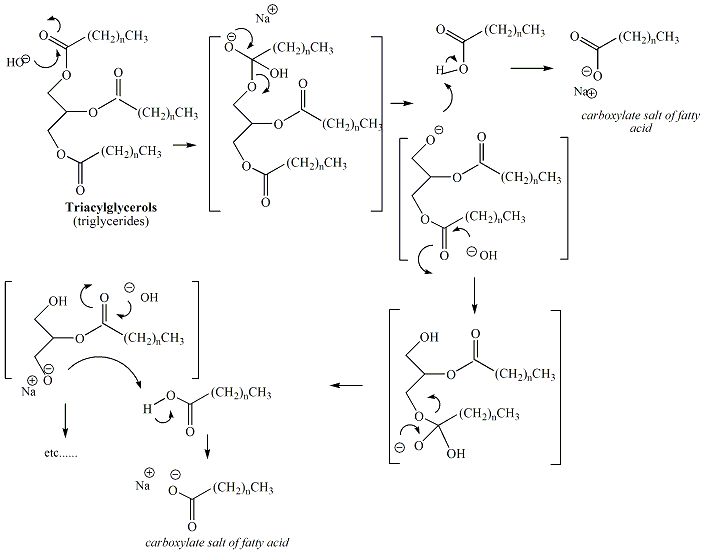
Base catalyzed hydrolysis of the ester functional groups of triacylglcerols, derived from fats and oils, results in the formation of soap. Soap is a mixture of carboxylate salts of fatty acids derived from the fatty acids of the triacylglycerols contained in the fat or oil. Typically, sodium or potassium hydroxide is used to prepare soap from fats and oils, resulting in sodium or potassium carboxylates. Figure 5 depicts the reaction scheme for conversion of a triacylglycerol to soap.
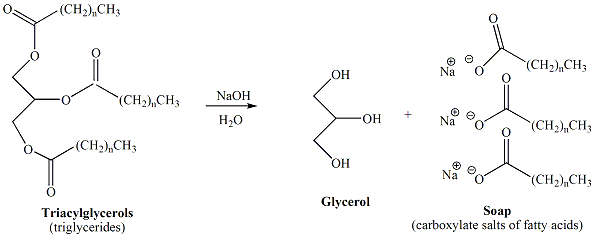
Figure 5
The mechanism of the reaction is a nucleophilic acyl
substitution. In the reaction to generate soap, three
sequential
hydrolysis reactions occur at each of the ester functional
groups of
the triacylglycerol. Figure 6 depicts the mechanism
of
saponification.
Properties of Soaps and Detergents
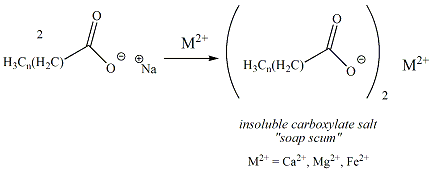
"Hard water" is water that contains numerous ions, especially calcium, magnesium and iron. Sodium and potassium carboxylates react with these hard water ions (reaction shown in Figure 9) to generate insoluble calcium , magnesium or ferric carboxylate salts that are insoluble in water. These insoluble carboxylate salts form a residue ("soap scum") that diminish the cleansing properties of soap.
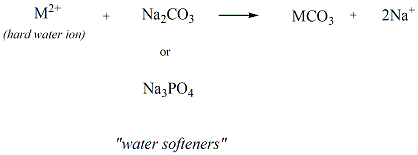
Emulsions
Emulsions are thermodynamically unstable two phase systems consisting of two immiscible (insoluble in each other) liquids. One of the liquids is referred to as the "dispersed phase" or "internal phase" that is distributed throughout the second phase which is referred to as the "continuous phase" or "external phase". Typically in an emulsion, one phase is hydrophilic (water or a very polar substance) and the other phase is lipophilic (oil or a very non-polar substance). There are two general types of emulsions; 1) oil in water emulsions (o/w) where the oil or nonpolar component is the dispersed phase and the water or polar substance is the continuous phase , and 2) water in oil emulsions (w/o) where the water or polar component is dispersed in the contious oil (non-polar) phase. The continuous phase typically constitutes >45% of the total weight of the emulsion. Oil in water emulsions are miscible in water and can be rinsed away with water. Examples of emulsions include salad dressing (oil and vinegar)when it is shaken. There are also numerous examples of emulsions in pharmaceutical preparations that include creams and lotions.
Emulsions form when these two immiscible liquids are aggitated, but are unstable as these "incompatible" phases tend to separate back (coalesce) to their original phases (or layers) over time. Emulsifiers (also referred to as surfactants) are used to stabilize emulsions. Emulsifiers have both a polar and non-polar component that allow them to concentrate at the interface between the polar and non-polar phses of the emulsion. Emulsyfing agents work by providing a protective barrier around a dispersed particle and prevent these particles from coalescing and reforming layers. Soaps and detergents are examples of emulsifiers. In the cleaning process, these agents form a barrier (micelle) around the dirt (dispersed phase). The micelles containing the trapped dirt particles have a polar surface and are miscible with water and capable of being washed away.
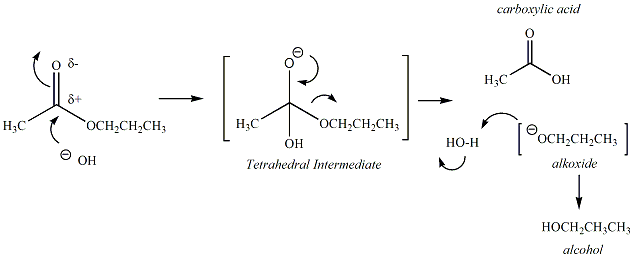
Esters are
carboxylic
acid dervatives that undergo hydrolysis under basic,
aqueous conditions
via a nucleophilic acyl substitution reaction.
Base-catalyzed
hydrolysis of esters is referred to as
"saponification". The
carbonyl carbon of the ester functional group reacts with
a hydroxide
nucleophile to generate a tetrahedral intermediate
(TI). The
carbonyl group is then regenerated to form a carboxylic
acid,
releasing an alkoxide as the leaving group. The
alkoxide
continues to react by abstracting a proton from water,
generating an
alcohol and regenerating more hydroxide to sustain
the
reaction. The products of the ester hydrolysis are a
carboxylic
acid and an alcohol. The mechanism of the ester
hydrolysis
reaction is given in Figure 1.
|
Lipids:
Fats and Oils
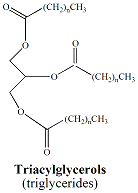
Lipids are a class of naturally-occuring, non-polar organic molecules. Fats and oils, also referred to as triacylglycerols, are two types of lipids that are characterized by the presence of ester functional groups that connect fatty acids to a glycerol backbone (Figure 2). Fatty acids are long chain hydrocarbons(commonly from 12 carbons up to 20 carbons) terminating in a carboxylic acid functional group. Typically, naturally-occurrring fatty acids are not branched and contain an even number carbons. There are two types of fatty acids; saturated fatty acids and unsaturated fatty acids. Saturated fatty acids contain no double bonds. Unsaturated fatty acids contain one or more double bonds that usually have a cis (Z) configuration. Polyunsaturated fatty acids contain multiple double bonds. A saturated fatty acid, palmitic acid (16-carbons) and an unsaturated fatty acid, palmitoleic acid (16 carbons, one double bond) and glycerol are shown in Figure 3. |
|
 Figure
2
|

Figure 3
The melting points
associated with saturated fatty acids are typically higher
than those
of unsaturated fatty acids of comparable carbon chain
length.
Saturated fatty acids have a regular, uniform structure
that allows
these molecules to pack tightly together in the solid
phase and
maintain close (strong) non-covalent interactions.
Unsaturated
fatty acids have less regular structures due to the
presence of the cis
double bond which limits the distance between molecules
resulting in
weaker non-covalent interactions (Figure 4).
Polyunsaturated
fatty acids have even less regular structures due to
multiple double
bonds. More energy is required to "break" the
stronger
non-covalent interactions associated with the saturated
fatty acids to
allow for a phase change from the solid phase to the
liquid phase,
resulting in higher melting points (See
a
similar discussion about
boiling points.). Table 1 provides a list of
common saturated
and
unstaturated fatty acids and their melting points.

Figure 4
| Fatty
Acid |
Number
of Carbons |
Melting
Point (0C) |
| Saturated |
||
| Lauric |
12 |
44 |
| Myristic |
14 |
58 |
| Palmitic |
16 |
63 |
| Stearic |
18 |
70 |
| Arachidic |
20 |
75 |
| Unsaturated |
||
| Palmitoleic |
16 |
32 |
| Oleic (one double bond |
18 |
16 |
| Linoleic (two double bonds) |
18 |
-5 |
| Arachidonic |
20 |
-50 |
Table 1: Melting
Points
of Common Fatty Acids
Triacylglycerols
contain
three fatty acid moeties. Fats and oils are made up
of complex
mixtures of triacylglycerols, each made up of a mixture of
saturated
and unsaturated fatty acids. The physical properties
associated
with specific fats and oils are determined by the
composition of its
triacylglycerols. Solid fats are typically composed
of a greater
percentage of saturated fatty acids (higher melting
points) relative to
liquid vegetable oils that contain more unsaturated
fatty acids
(lower melting points). Table 2 lists the
composition (in
terms of fatty acids) of some common fats and oils.
Saponification:
Synthesis
of Soap| Saturated
(%) |
Unsaturated
(%) |
Other (%) |
|||||
| Source |
Lauric (C12) |
Myristic (C14) |
Palmitic (C16) |
Stearic (C18) |
Oleic (C18) |
Linoleic (C18) |
--- |
| Fats |
|||||||
| Lard |
--- |
1 |
25 |
15 |
50 |
6 |
8 |
| Butter |
2 |
10 |
25 |
10 |
25 |
5 |
23 |
| Human Fat |
1 |
3 |
25 |
8 |
46 |
10 |
12 |
| Oils |
|||||||
| Corn |
--- |
1 |
10 |
4 |
35 |
45 |
5 |
| Olive |
--- |
1 |
5 |
5 |
80 |
7 |
2 |
| Peanut |
--- |
--- |
7 |
5 |
60 |
20 |
8 |
Base catalyzed hydrolysis of the ester functional groups of triacylglcerols, derived from fats and oils, results in the formation of soap. Soap is a mixture of carboxylate salts of fatty acids derived from the fatty acids of the triacylglycerols contained in the fat or oil. Typically, sodium or potassium hydroxide is used to prepare soap from fats and oils, resulting in sodium or potassium carboxylates. Figure 5 depicts the reaction scheme for conversion of a triacylglycerol to soap.

Figure 5

Figure 6:
Mechanism of
Saponification
The reaction to make soap involves heating the fat and/or oil with sodium or potassium hydroxide in water. Upon completion of the hydrolysis, the carboxylate salts are isolated from the sodium or potassium hydroxide and glycerol by precipitation in cold sodium chloride. The carboxylate salts are then filtered and washed to provide the purified soap. (Click here to read more about the history of soapmaking)
The reaction to make soap involves heating the fat and/or oil with sodium or potassium hydroxide in water. Upon completion of the hydrolysis, the carboxylate salts are isolated from the sodium or potassium hydroxide and glycerol by precipitation in cold sodium chloride. The carboxylate salts are then filtered and washed to provide the purified soap. (Click here to read more about the history of soapmaking)
Properties of Soaps and Detergents
| Soaps
and
detergents function as cleaning agents by
organizing into micelles that trap non-polar dirt
and oil
particles.
Carboxylate salts of soaps form micelles,
spherical structures in which
the polar carboxylate is oriented on the outside
diameter of the sphere
and the non-polar hydrocarbon chains of the fatty
acids are oriented
into the center of the sphere. Dirt and oil
associate with the
non-polar tails of the carboxylate salts ("like
dissolves like")
through hydrophobic interactions and are trapped
in the center of the
sphere. Since the outer surface of the micelle is
polar, it is water
soluble and can be washed or rinsed out with water
(polar solvent).
Figure 7 depicts the structure of a micelle
illustrating how dirt and
other non-polar particles are trapped in the
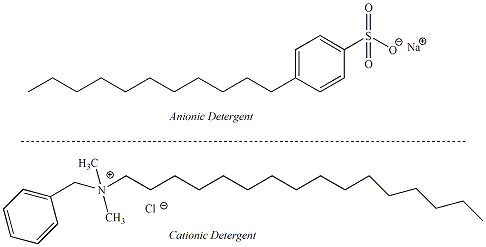
center. Detergents are similar to soaps in their properties but use alternative functional groups as the polar end of the molecule. Detergents are considered to be "synthetic" cleansing agents because they are not dervied from naturally occurring fats and oils. The non-polar part of the detergent molecule may be a branched hydrocarbon or a straight-chain hydrocarbon. Detergents that contain straight chain hydrocarbons are preferred as these are biodegradable. Anionic detergents are derived from sulfonic acids and form sulfonate salts when ionized. Ammonium salts attached to non-polar hydrocarbons are examples of cationic detergents. Figure 8 shows the structures of some synthetic detergents. |
|
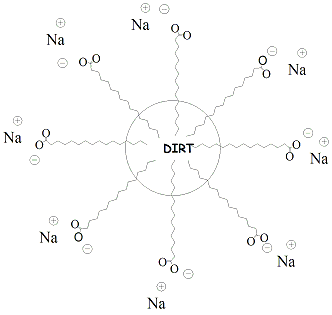 Figure 7: Depiction of a Micelle |

Figure 8:
Structures of Some
Common Synthetic Detergents
Soaps have numerous characteristics that can be
enhanced by
selecting certain types of fats and oils as starting
materials and
through careful design of the soap formulation. The
hardness of
the soap is determined by the percent composition of
saturated fatty
acids. Saturated fatty acids (higher melting points)
provide
harder soaps while higher unsaturated fatty acid content
result in
softer soaps. Also, potassium carboxylates
generally result in softer soaps
relative to sodium carboxylates. The cleansing
properties of the
soap are related to their ability to effectively form
stable micelles
and trap dirt."Hard water" is water that contains numerous ions, especially calcium, magnesium and iron. Sodium and potassium carboxylates react with these hard water ions (reaction shown in Figure 9) to generate insoluble calcium , magnesium or ferric carboxylate salts that are insoluble in water. These insoluble carboxylate salts form a residue ("soap scum") that diminish the cleansing properties of soap.

Figure
9: Formation of Soap Scum by Reaction of Soap with
Hard Water
Water softeners are used to minimize the formation
of soap
scum by reacting with the hard water ions to generate
water soluble
salts. Sodium carbonate and sodium phosphate
tribasic are two
compound that are commonly used as water softeners.
Figure 10
shows the reaction that occurs to generate the water
soluble salts
generated from the hard water ions and these water
softeners. 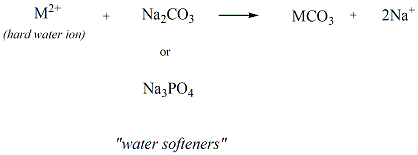
Figure 10: Water
Softeners
Emulsions
Emulsions are thermodynamically unstable two phase systems consisting of two immiscible (insoluble in each other) liquids. One of the liquids is referred to as the "dispersed phase" or "internal phase" that is distributed throughout the second phase which is referred to as the "continuous phase" or "external phase". Typically in an emulsion, one phase is hydrophilic (water or a very polar substance) and the other phase is lipophilic (oil or a very non-polar substance). There are two general types of emulsions; 1) oil in water emulsions (o/w) where the oil or nonpolar component is the dispersed phase and the water or polar substance is the continuous phase , and 2) water in oil emulsions (w/o) where the water or polar component is dispersed in the contious oil (non-polar) phase. The continuous phase typically constitutes >45% of the total weight of the emulsion. Oil in water emulsions are miscible in water and can be rinsed away with water. Examples of emulsions include salad dressing (oil and vinegar)when it is shaken. There are also numerous examples of emulsions in pharmaceutical preparations that include creams and lotions.
Emulsions form when these two immiscible liquids are aggitated, but are unstable as these "incompatible" phases tend to separate back (coalesce) to their original phases (or layers) over time. Emulsifiers (also referred to as surfactants) are used to stabilize emulsions. Emulsifiers have both a polar and non-polar component that allow them to concentrate at the interface between the polar and non-polar phses of the emulsion. Emulsyfing agents work by providing a protective barrier around a dispersed particle and prevent these particles from coalescing and reforming layers. Soaps and detergents are examples of emulsifiers. In the cleaning process, these agents form a barrier (micelle) around the dirt (dispersed phase). The micelles containing the trapped dirt particles have a polar surface and are miscible with water and capable of being washed away.