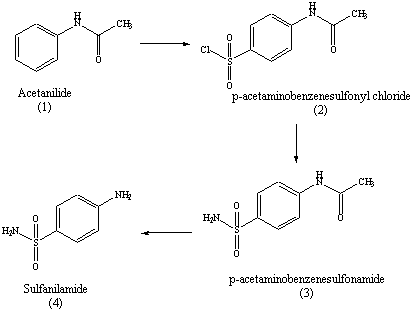
Figure 4.1: Synthetic Scheme for Preparation of Sulfanilamide

Figure 4.1: Synthetic Scheme for Preparation of Sulfanilamide
Acetanilide (1) will be used as the starting material.
Reaction
of acetanilide with chlorosulfonic acid provides
p-acetaminobenzene
sulfonyl
chloride (2) through an electrophilic aromatic substitution.
The
sulfonyl chloride reacts readily with ammonia in a reaction that
is
mechanistically
analogous to the nucleophilic acyl substitution reaction of
carbonyl
compounds.
The resulting product of this step of the reaction is
p-acetaminobenzenesulfonamide
(3). Selective acid-catalyzed hydrolysis of the amide (but
not
the
sulfonamide) occurs to provide sulfanilamide (4) (and acetic acid)
through
a nucleophilic acyl substitution reaction. Click here for details about the
specific
mechanisms for
each
of the steps described in Figure 4.1.
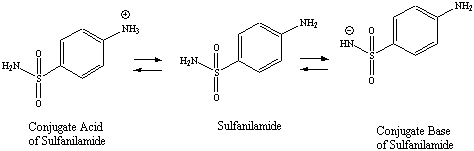
Figure 4.2: Ionization Scheme for Sulfanilamide
Isolation of the sulfanilamide product from the reaction mixture can be elusive since the compound contains two ionizable functional groups, one acidic and one basic (Figure 4.2). The reaction mixture must be brought to an appropriate pH in which the compound is in its unionized form, where it can be isolated from the polar, aqueous reaction medium in good yield.
Once the drug product is isolated, it will be weighed and a percent yield will be calculated using acetanilide as the limiting reagent. Characterization of the product will be done by determining the melting point of the isolated product and comparing it to the literature value reported for sulfanilamide. Finally, buffered solutions of the product will be prepared for use in a microbiological analysis to evaluate the antimicrobial activity of the synthesized sulfanilamide.
Procedure (Week 1)
Weigh out 5.0g of acetanilide and dispense it into a 125 ml
Erlenmeyer
flask [See
video]. Add a
magnetic stir bar to the flask. Clamp
the
flask to a ring stand and set it in an ice bath resting on a hot
plate/stirrer.
Position the mouth of the flask under a burette containing the
chlorosulfonic
acid [See
video]. See Figure 4.3 for
an illustration of the set-up.
Turn
on the stirrer and slowly open the stopcock of the burette to
dispense
12.5 ml of chlorosulfonic acid, drop by drop, into the Erlenmeyer
flask.
(Warning:
Chlorosulfonic acid is extremely corrosive and toxic.
Gloves must
be worn!) Continue stirring the reaction mixture as
much as
possible
until all the chlorosulfonic acid is added [See video].
Figure 4.3: Set-up for addition of chlorosulfonic acid to acetanilide
While the chlorosulfonic acid is being added to the flask, clamp
a
water
trap to a second ring stand. When the addition of 12.5ml of
chlorosulfonic
acid is complete, connect the reaction flask to a vacuum trap
filled
with
~30ml of water, keeping both flasks clamped to their
respective
ring
stands. The glass tube inserted into the vacuum trap should
be
positioned
~1 inch above the surface of the water. Insert the
rubber
stopper
at the end of the trap hose into the mouth of the reaction flask.
Remove
the ice bath (if it is plastic) from the reaction flask and place
it
under the trap
flask.
Place a new, glass water bath under the reaction flask. If the ice
bath
is glass, leave it in place and place a new ice bath under the
flask
used as the trap. See Figure 4.4
for
an illustration of the proper set-up. Continue stirring the
reaction
mixture and unclamp the reaction flask if necessary to swirl its
contents.
Stir until all of the acetanilide is dissolved [See video].
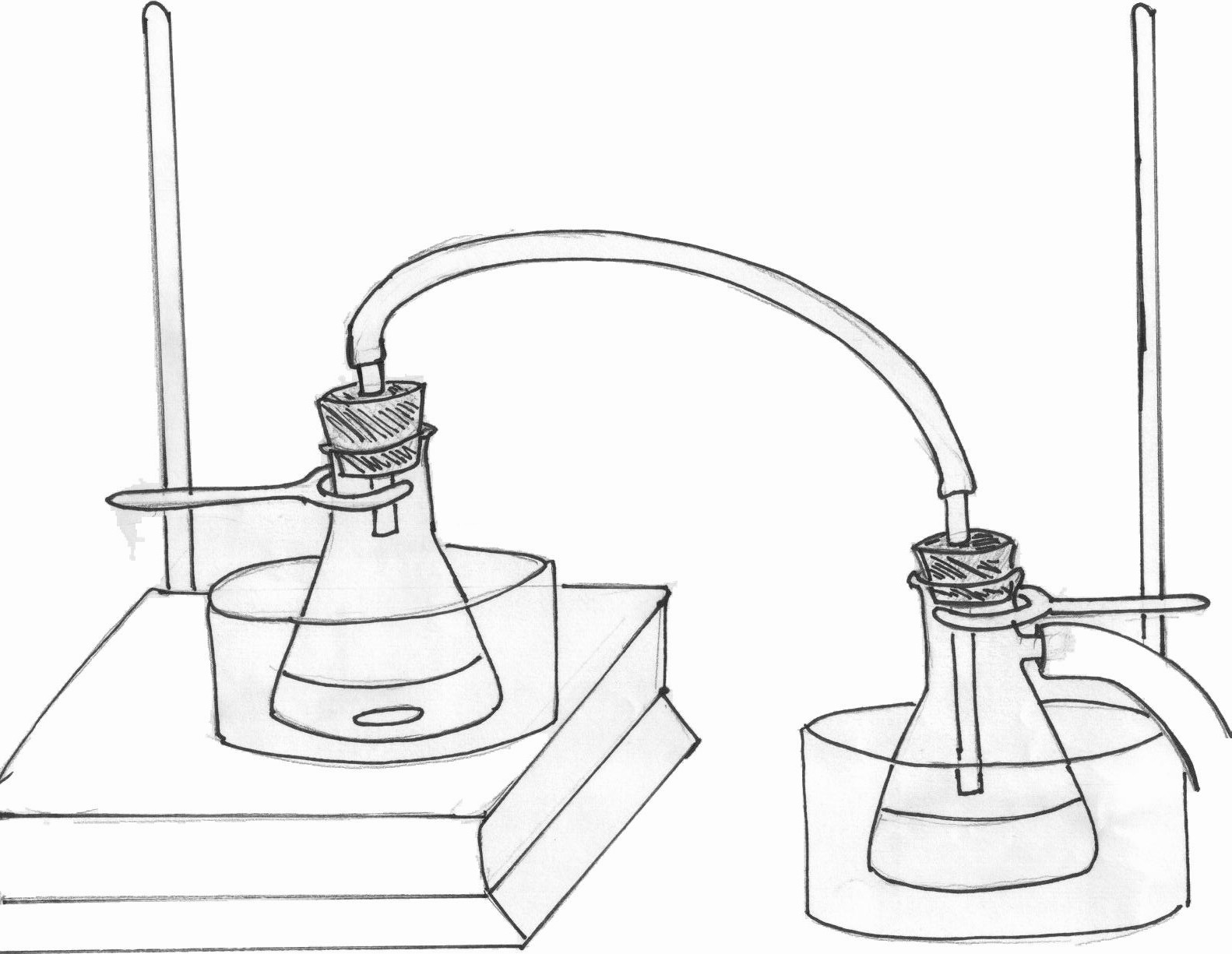
Figure 4.4: Reaction flask attached to gas trap
After the acetanilide is completely dissolved, turn on the water bath to just below boiling and heat the reaction mixture for 10 minutes. After the heating is complete, a brown-yellow oil should remain. Add ~75ml of ice to a 150ml beaker. Disconnect the trap from the reaction flask containing the oil product. Using a disposable glass pipette, add all the oil to the beaker containing the ice, drop by drop. As the oil comes in contact with the ice, a white precipitate should form [See video]. Add an additional 10ml of cold, distilled water to the beaker and swirl the mixture until all the ice is melted. Separate the solid from the mixture using vacuum filtration.
Clamp a 125 ml vacuum flask, fitted with a Buchner funnel and
filter
paper, to a ring stand. Attach a vacuum hose to the
flask
and
aspirator vacuum. Turn on the aspirator and pour the
contents of
the beaker through the Buchner funnel, collecting the solid on
top.
Wash the solid twice with ~20 ml of cold, distilled water.
The
solid
is p-acetaminobenzenesulfonyl chloride [See video].
Transfer the p-acetaminobenzene sulfonyl chloride to a clean 125
ml
Erlenmeyer flask. Add a magnetic stir bar to the
flask.
Clamp
the flask to a ring stand and set it in a water bath resting on a
hot
plate/stirrer.
Add 15ml of ammonia solution and
15 ml of distilled water to the flask. Begin stirring the
reaction
mixture and heat the water bath to boiling for 5-10 minutes.
The
the consistency of the suspension will become more "pasty" as the
reaction
progresses [See
video]. Turn off the heat and remove the water
bath.
Place
the flask in an ice bath and allow the reaction mixture to cool
thoroughly
(~5-7 minutes). Isolate the reaction product by vacuum
filtration.
Clamp a 125 ml vacuum flask, fitted with a Buchner funnel and filter paper, to a ring stand. Attach a vacuum hose to the flask and aspirator vacuum. Turn on the aspirator and pour the contents of the reaction flask through the Buchner funnel, collecting the solid on top [See video]. Wash the solid twice with ~20 ml of cold, distilled water. The solid is p-acetaminobenzenesulfonamide. Transfer the solid to a 50 ml beaker labeled with your name and "p-acetaminobenzenesulfonamide". Place the beaker in your lab drawer until the second week of the experiment.
If a solid precipitates from the solution.....
The solid sulfanilamide may precipitate from the solution after
the
pH has been adjusted to 7. Filter the solid using vacuum
filtration [See video].
Transfer the solid to a watch glass to dry. Save the
filtrate
until
a melting point determination has been done to verify that the
solid
has
the same melting point as commerical sulfanilamide.
If no solid precipitates from the solution.....
If no solid precipitates from the solution after the pH has been
adjusted
to ~7, heat the flask to boil off the water from the
solution.
Continue
heating until approximately 20-30ml of water remains. Cool
the
flask
in an ice bath to promote precipitation of the solid produc from
the
solution.
Filter the solid using vacuum filtration. Transfer the solid
to a
watch glass to dry. Save the filtrate until a melting point
determination
has been done to verify that the solid has the same melting point
as
commerical
sulfanilamide.
Weight and Melting Point Determination of Sulfanilamide
Inspect your solid product and if it appears to be dry, weigh it
and
calculate the percent yield. Calibrate the thermometer of
the
melting
point apparatus with benzoic acid (lit mp = 122oC).
Record the melting point of the product [See
video].
TLC Analysis of Sulfanilamide
Prepare a TLC plate with two tick marks (labeled 1 and 2) to
analyze
commercial sulfanilamide (1) and your synthesized sulfanilamide
(2).
Use the EtOH solution from the vial labeled
"sulfanilamide"
for the TLC analysis. Prepare a solution of your product in
ethanol
(10mg product/1-2ml EtOH). Spot the plate with the solutions
using
two capillary pipettes and allow the spots to dry completely
before
developing
the plate. Develop the plate using a developing solvent that
will
provide an Rf of ~0.5. Start with 30:70 ethanol: hexane and
make
appropriate adjustments in the developing solvent ratio, if
necessary.
View the plate under UV light and in the iodine chamber.
Record
your
results.
IR Analysis of Sulfanilamide
Run an IR spectrum of the product. See IR
Spectroscopy for a review of sample preparation and
interpretation
of IR spectra [See video].
Microbiologic
Analysis
of Product